Nashville Veterinary Specialists
“Atypical” Hypoadrenocorticism- Recognition and Management of an Enigmatic Condition
Information provided by Julie Stegeman, DVM, DACVIM, Nashville Veterinary Specialists - Clarksville
Hypoadrenocorticism, a.k.a. “Addison’s Disease” is the failure of the adrenal cortices to produce adequate hormone levels. The classic version of Addison’s refers to loss of production of both glucocorticoids and mineralocorticoids. This is usually due to immune mediated destruction of the adrenal cortical tissue. These patients are also referred to as “Primary” Addison’s cases, as the adrenal gland itself is the source of the problem.
“Secondary” Addison’s refers to loss of ACTH secretion by the pituitary gland, thus leading to loss of production of glucocorticoids only by the adrenal glands. Mineralocorticoid secretion is not affected.
“Atypical” Addison’s is a term that includes all patients with Addison’s disease that present with normal electrolytes at the time of diagnosis. Most of these patients are still Primary cases, of which at least half may become “typical” in time and develop the expected electrolyte abnormalities. A few “Atypical” patients have Secondary Addison’s, and therefore remain only glucocorticoid deficient.
In the past two decades there has been increasing awareness of the existence of Atypical Addison’s, and increased testing for the condition. Depending on the reference, Atypical cases can comprise anywhere from 3% to 23% of the Addison’s cases diagnosed. Atypical Addison’s may also be referred to as “glucocorticoid deficient hypoadrenocorticism” in the literature.
It is extremely important to differentiate which form of the condition is present, to ensure correct treatment. This article will specifically discuss Atypical Addison’s disease, including recognition of suspected cases, diagnosis and management. Iatrogenic Addison’s due to over-response to therapy for Cushing’s disease will not be covered herein.
Signalment
Hypoadrenocorticism has the highest incidence in middle aged female dogs regardless of which form is evaluated. There is a breed predisposition for Standard Poodles, Portuguese Water Dogs, West Highland White Terriers, Great Danes, Rottweilers, Bearded Collies, Duck Tolling Retrievers, and Wheaten Terriers. Intact females have higher risk than spayed females, which have greater risk than neutered males, followed by intact males. Atypical Addison’s patients tend to be older (median 7 yr) compared to “typical” cases (median 4.4 years).
Clinical Signs
Glucocorticoids such as cortisol are responsible for supporting normal blood pressure, muscle strength, glucose production, GI tract motility and blood flow, and they facilitate the response to catecholamines. A lack of cortisol leads to weakness, vomiting, diarrhea, lethargy, and inappetance. In addition, neuromuscular signs such as tremors, partial seizure, cramping, lameness, regurgitation, or even full hypoglycemic seizures can occur. Dogs with Atypical Addison’s tend to have a longer duration of clinical signs (1-2 months) prior to diagnosis as compared to “typical” cases (days to weeks).
Mineralocorticoids such as aldosterone are responsible for sodium/chloride/water reclamation and potassium excretion in the distal nephron, so lack of this hormone leads to hypotension, possibly bradycardia, and neuromuscular weakness. In Atypical Addison’s disease, electrolyte balance is usually normal, due to preserved mineralocorticoid function. Therefore, some of the symptoms may be lacking or less overt with Atypical Addison’s patients. Of note, since ACTH is an inhibitor of vasopressin, its lack in Secondary Addison’s may cause excessive vasopressin secretion, leading to water retention and mild dilutional hyponatremia.
Minimum Data Base and Imaging
The classic combination of hyponatremia and hyperkalemia is the usual “red flag” to consider Addison’s disease as a possibility. However, Atypical Addison’s patients will not have those electrolyte changes. Azotemia is also less common in Atypical Addison’s due to preserved mineralocorticoid function, but does occur. The azotemia is usually pre-renal and reversible. The urine specific gravity may not exceed 1.030 due to some degree of medullary washout.
Cortisol deficiency will cause an inability to generate a stress leukogram, so lack of neutrophilia or lymphopenia in a stressed patient may be noted. A mild to moderate non-regenerative anemia is often present, as a result of impaired bone marrow production coupled with GI losses.
Cortisol facilitates gluconeogenesis and decreases peripheral utilization of glucose. Hypocortisolemia can cause hypoglycemia that is severe enough to cause seizures. Cortisol also facilitates fat and protein absorption, and protein production. The presence of a triad of hypoglycemia, hypocholesterolemia, and hypoalbuminemia should alert the clinician to consider a diagnosis of Atypical Addison’s. The other differentials for that triad are protein losing enteropathy and hepatic failure.
Hepatic enzyme elevations may occur due to impaired hepatic blood flow, and in some cases may be due to concurrent immune mediated hepatopathy such as chronic active hepatitis. This author has also seen a case of hepatic lipidosis associated with secondary Addison’s disease in a dog.
Thoracic radiographs may show microcardia and a small vena cava if obtained during an Addisonian crisis. There may be evidence of megaesophagus in some patients, possibly with concurrent aspiration pneumonia. The megaesophagus can be due to the effects of glucocorticoid deficiency, or in rare cases, may reflect concurrent myasthenia gravis.
Abdominal ultrasound will usually show very small adrenal glands. In our hospital, adrenal ultrasonography is commonplace, so difficulty finding the adrenal glands may prompt submittal of cortisol levels where appropriate.
Definitive Diagnosis
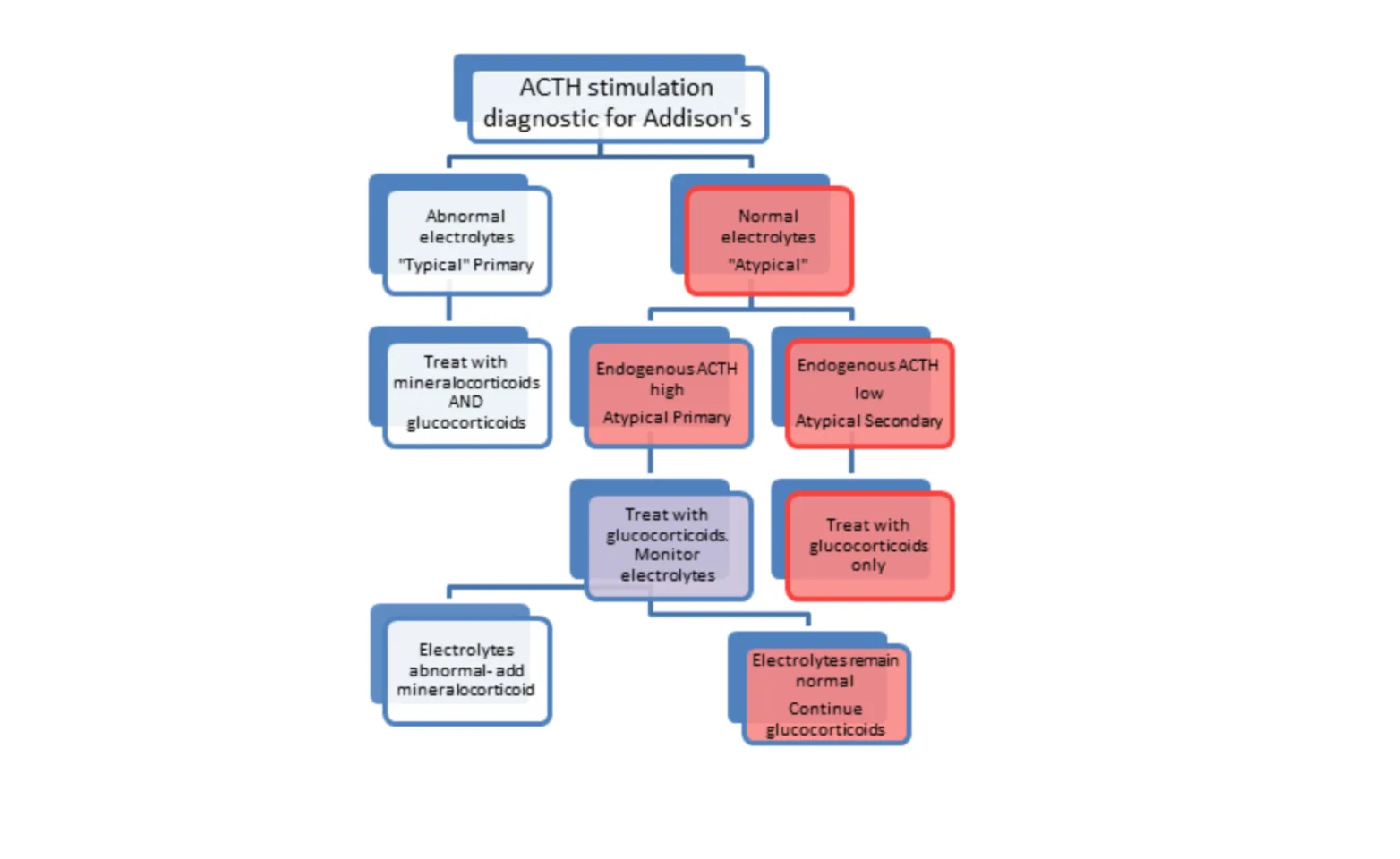
A resting cortisol level ≥ 2.0 mcg/dl is considered a cutoff for ruling out Addison’s disease. Said another way, if the resting cortisol is < 2.0 mcg/dl, then an ACTH stimulation test is advised. Addison’s disease can ONLY be definitively diagnosed via a subnormal cortisol response to ACTH stimulation. The resting cortisol level is most accurate when utilizing reference laboratories instead of an in house analyzer.
There are multiple protocols for the ACTH stimulation test, which can utilize a synthetic ACTH gel or brand name Cortrosyn® suspension. Accuracy of the test is affected by exposure to exogenous corticosteroids, including topical therapies. A careful history must be taken to exclude exposure to exogenous glucocorticoids when interpreting the test results. If a patient has Addison’s disease, the baseline cortisol level and the 1 hour post ACTH cortisol level will both be subnormal, and often undetectable.
If “atypical” Addison’s disease is diagnosed in a patient with normal electrolytes, an endogenous ACTH level can be performed on the baseline blood sample from the ACTH stimulation test. Since special handling is required for endogenous ACTH levels, the sample should be obtained intentionally and set aside for possible submittal in cases where Atypical Addison’s is suspected.
The endogenous ACTH level will be high if the patient has primary Addison’s, and thus has not yet manifested a mineralocorticoid deficiency. The majority of atypical cases are actually primary, and of those, only up to half will ever require mineralocorticoid therapy. These patients should have electrolytes measured regularly to watch for a transition to more “typical” Addison’s. That transition can occur within days to weeks in most cases. This author advises electrolytes every 2 weeks for 2 months, then monthly for 4-6 months, then twice a year thereafter.
The endogenous ACTH will be very low in secondary Addison’s, as the pituitary is failing to secrete that hormone. These patients will only ever require glucocorticoid therapy, and electrolyte monitoring should not be as critical once the crisis is passed. However, monitoring of a minimum data base every 3-6 months is advisable in any Addisonian patient.
One can also run aldosterone levels before and 60-90 minutes after ACTH injection. However, the results can be quite variable and do not necessarily accurately predict the future need for mineralocorticoid support. The aldosterone levels can be quite low and the patient may still maintain normal sodium and potassium levels. It is the electrolyte levels that should determine whether to supplement mineralocorticoids.

Treatment
Treatment must begin with supportive care proportionate to the patient’s clinical abnormalities. This usually involves intravenous fluid therapy, +/- dextrose supplementation, antiemetics, antacids etc.
ALL DOGS WITH ADDISON’S DISEASE NEED GLUCOCORTICOID SUPPLEMENTATION! Dogs with secondary and “atypical” Addison’s ONLY need glucocorticoid support. These patients should NOT receive DOCP or fludrocortisone.
Glucocorticoid supplementation is usually achieved with prednisone or dexamethasone. Dexamethasone may technically be preferred since it has minimal mineralocorticoid effects. However, use of prednisone is much more common. Doses for prednisone range from 0.1-0.2 mg/kg/day to start with. If using dexamethasone, 1/7th of the calculated prednisone dose should be used. Often the glucocorticoid can be reduced in dose, and reduced in frequency to every other day. When in the throes of an Addisonian crisis, intravenous Dexamethasone SP is advised, at an anti-inflammatory dose (supra-physiologic) for the first several days.
Treatment continued:
Signs of inadequate glucocorticoid supplementation include lethargy, inappetance, vomiting, or diarrhea. Signs of excessive glucocorticoid supplementation include polydipsia, polyuria, polyphagia, thinning coat, weight gain, and panting. If blood work shows anemia, hypoalbuminemia, hypocholesterolemia, then a higher dose of glucocorticoid may be needed. Likewise, if there is an elevated ALKP and dilute urine with proteinuria, it is possible that the glucocorticoid dose may be too high. The dose of glucocorticoid will need adjustment over the lifespan of the pet. Owners are advised to give twice the usual dose on days of unusual stress such as boarding, grooming, fireworks, veterinary visits, etc.
Mineralocorticoid supplementation is provided ONLY for “typical” primary Addison’s patients…i.e. ONLY for those exhibiting electrolyte disturbances. DOCP (desoxycorticosterone pivalate, Percorten-V®) is currently the most common and most reliable drug for this purpose.
Prognosis
Once “atypical” Addison’s disease is recognized and the diagnosis is confirmed, prognosis is excellent in most cases. The challenge lies in recognizing the condition, and then accurately confirming the diagnosis. The treatment can then be tailored to the specific deficiencies that the patient has, and adjusted according to individual response to therapy. This can be an extremely rewarding disease to treat, as the patients can make remarkable recoveries.
References
Peterson M, Kintzer P, Kass P. Pretreatment clinical and laboratory findings in dogs with hypoadrenocorticism: 225 cases (1979-1993). J Am Vet Med Assoc 1996; 208: 85-91.
Lifton S, King L, Zerbe C. Glucocorticoid deficient hypoadrenocorticism in dogs: 18 cases (1986-1995). J Am Vet Med Assoc 1996; 209: 2076-2081.
Feldman E, Nelson R. Hypoadrenocorticism. In: Canine and feline endocrinology and reproduction, 2nd Ed. Philadelphia, Pa: WB Saunders, 1996;266-302
Greco D. Hypoadrenocorticism in dogs and cats. Vet Med 2000; pp 468- 476.
Oberbauer A, Benemann K, Belanger J et al. Inheritance of hypoadrenocorticism in Bearded Collies. Am J Vet Res 2002; 63:643-647.
Feldman E, Nelson R. Hypoadrenocorticism (Addison’s Disease). In:Canine and feline endocrinology and reproduction, 3rd Ed. Philadelphia, Pa: WB Saunders, 2004; 394- 439.
Lathan P, Tyler J. Canine hypoadrenocorticism: pathogenesis and clinical features. Compend Contin Educ Pract Vet 2005; 110-120.
Lathan P, Tyler J. Canine hypoadrenocorticism: diagnosis and treatment. Compend Contin Educ Pract Vet 2005; 121-130.
Adler J, Drobatz K, Hess R. Abnormalities of serum electrolyte concentrations in dogs with hypoadrenocorticism. J Vet Intern Med 2007; 21: 1168- 1173.
Thompson A, Scott-Moncrief J, Anderson J. Comparison of classic hypoadrenocorticism with glucocorticoid- deficient hypoadrenocorticism in dogs: 46 cases (1985- 2005). J Am Vet Med Assoc 2007; 230: 1190-1194.
Hughes A, Nelson R, Famula T et al. Clinical Features and heritability of hypoadrenocorticism in Nova Scotia Duck Tolling Retrievers: 25 cases (1994-2006). J Am Vet Med Assoc 2007; 231: 407-412.
Lennon E, Boyle T, Hutchins R et al. Use of basal serum or plasma cortisol concentrations to rule out a diagnosis of hypoadrenocorticism in dogs: 123 cases (2000-2005). J Am Vet Med Assoc 2007; 231: 413-416.
Scott-Moncrief J. Diagnostic Testing for Canine Hypoadrenocorticism; What’s New?. In: Proceedings: American College of Veterinary Internal Medicine Forum; 6/5/2008
Gaydos C, DeClue A. Updates on canine hypoadrenocorticism. Vet Med 2008; 320-332.
Seth M, Drobatz K, Church D, et al. White blood cell count and the sodium to potassium ratio to screen for hypoadrenocorticism in dogs. J Vet Intern Med 2011; 25: 1351-1356.
